Chemistry-
General
Easy
Question
The van der Waals constant for gases and
and  are as follows:
are as follows:

Which gas has the highest critical temperature?



- None
The correct answer is: 
Since, 
Hint: Gas  has the highest value of a, therefore, it has the highest critical temperature
has the highest value of a, therefore, it has the highest critical temperature
Related Questions to study
chemistry-
The distribution of the molecular velocities of gas molecules at any temperature  is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)
is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)

Where
 is molecular velocity
is molecular velocity
 is number of molecules having velocity
is number of molecules having velocity 
Let us define  , which is equal to the number of molecules between the velocity range
, which is equal to the number of molecules between the velocity range  and
and  , given by
, given by

Where
 is total number of molecules
is total number of molecules
 and
and 
 is universal gas constant
is universal gas constant
 is temperature of the gas
is temperature of the gas
 is molecular weight of the gas
is molecular weight of the gas
SI units of  are
are
The distribution of the molecular velocities of gas molecules at any temperature  is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)
is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)

Where
 is molecular velocity
is molecular velocity
 is number of molecules having velocity
is number of molecules having velocity 
Let us define  , which is equal to the number of molecules between the velocity range
, which is equal to the number of molecules between the velocity range  and
and  , given by
, given by

Where
 is total number of molecules
is total number of molecules
 and
and 
 is universal gas constant
is universal gas constant
 is temperature of the gas
is temperature of the gas
 is molecular weight of the gas
is molecular weight of the gas
SI units of  are
are
chemistry-General
chemistry-
The figure given below shows three glass chambers that are connected by valves of negligible volume. At the outset of an experiment, the valves are closed and the chambers contain the gases as detailed in the diagram. All the chambers are at the temperature of 300 K and external pressure of 1.0 atm
 atm
atm

What will be the work done by  gas when valve 2 is opened and value 1 remains closed?
gas when valve 2 is opened and value 1 remains closed?
The figure given below shows three glass chambers that are connected by valves of negligible volume. At the outset of an experiment, the valves are closed and the chambers contain the gases as detailed in the diagram. All the chambers are at the temperature of 300 K and external pressure of 1.0 atm
 atm
atm

What will be the work done by  gas when valve 2 is opened and value 1 remains closed?
gas when valve 2 is opened and value 1 remains closed?
chemistry-General
chemistry-
In simple cubic lattice, the spheres are packed in the form of a square array by laying down a base of spheres and then piling upon the base other layers in such a way that each sphere is immediately above the other sphere. In this structure, each sphere is in contact with six nearest neighbours. The percentage of occupied volume in this structure can be calculated as follows
The edge length ‘ ’ of the cube will be twice the radius of the sphere,
’ of the cube will be twice the radius of the sphere,  . Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is
. Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is

 or
or 
The fraction of the total volume occupied by the sphere is

In a simple cubic cell, an atom at the corner contributes to the unit cell
In simple cubic lattice, the spheres are packed in the form of a square array by laying down a base of spheres and then piling upon the base other layers in such a way that each sphere is immediately above the other sphere. In this structure, each sphere is in contact with six nearest neighbours. The percentage of occupied volume in this structure can be calculated as follows
The edge length ‘ ’ of the cube will be twice the radius of the sphere,
’ of the cube will be twice the radius of the sphere,  . Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is
. Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is

 or
or 
The fraction of the total volume occupied by the sphere is

In a simple cubic cell, an atom at the corner contributes to the unit cell
chemistry-General
chemistry-
Sketch shows the plot of Zvsp for a hypothetical gas for one mole at
three distinct temperature


Boyle’s temperature is the temperature at which a gas shows ideal behaviour over a pressure range in the low pressure region. Boyle’s temperature  . If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as
. If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as  and the temperature above critical temperature curve is more like
and the temperature above critical temperature curve is more like at
at 
For 500 K plot value of  changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of
changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of  will be
will be
Sketch shows the plot of Zvsp for a hypothetical gas for one mole at
three distinct temperature


Boyle’s temperature is the temperature at which a gas shows ideal behaviour over a pressure range in the low pressure region. Boyle’s temperature  . If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as
. If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as  and the temperature above critical temperature curve is more like
and the temperature above critical temperature curve is more like at
at 
For 500 K plot value of  changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of
changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of  will be
will be
chemistry-General
physics-
In double slit experiment, the distance between two slits is 0.6 mm and these are illuminated with light of wavelength 4800 A0 The angular width of dark fringe on the screen at a distance 120 cm from slits will be
In double slit experiment, the distance between two slits is 0.6 mm and these are illuminated with light of wavelength 4800 A0 The angular width of dark fringe on the screen at a distance 120 cm from slits will be
physics-General
chemistry-
I, II, and III are three isotherms, respectively, at  and
and  . Temperature will be in order
. Temperature will be in order

I, II, and III are three isotherms, respectively, at  and
and  . Temperature will be in order
. Temperature will be in order

chemistry-General
chemistry-
 vs
vs  curves at different pressures
curves at different pressures  and
and  for an ideal gas are shown below:
for an ideal gas are shown below:

Which one of the following is correct?
 vs
vs  curves at different pressures
curves at different pressures  and
and  for an ideal gas are shown below:
for an ideal gas are shown below:

Which one of the following is correct?
chemistry-General
physics-
In a YDSE shown in Fig a parallel beam of light is incident on the slits from a medium of refractive index n1 The wavelength of light in this medium is l1 A transparent slab of thickness ‘t’ and refractive index n3 is put in front of one slit The medium between the screen and the plane of the slits is n2 The phase difference between the light waves reaching point “O” (symmetrical, relative to the slits) is

In a YDSE shown in Fig a parallel beam of light is incident on the slits from a medium of refractive index n1 The wavelength of light in this medium is l1 A transparent slab of thickness ‘t’ and refractive index n3 is put in front of one slit The medium between the screen and the plane of the slits is n2 The phase difference between the light waves reaching point “O” (symmetrical, relative to the slits) is

physics-General
physics-
In Young’s double slit experiment  are two slits Films of thickness
are two slits Films of thickness  and refractive indices
and refractive indices  are placed in front of
are placed in front of  respectively If
respectively If  , then the central maximum will
, then the central maximum will

In Young’s double slit experiment  are two slits Films of thickness
are two slits Films of thickness  and refractive indices
and refractive indices  are placed in front of
are placed in front of  respectively If
respectively If  , then the central maximum will
, then the central maximum will

physics-General
chemistry-
Actual graph for the given parameters. For the non-zero volume of the molecules, real gas equation for  mol of the gas will be
mol of the gas will be

Actual graph for the given parameters. For the non-zero volume of the molecules, real gas equation for  mol of the gas will be
mol of the gas will be

chemistry-General
chemistry-
For 1 mol of an ideal gas,  in Fig.
in Fig.
I)  in Fig.
in Fig.
II)  in Fig.
in Fig.
III) and  in Fig.
in Fig.
IV) then which curves are correct
I) 
II) 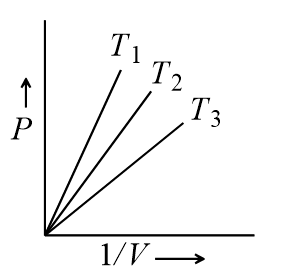
III) 
IV) 
For 1 mol of an ideal gas,  in Fig.
in Fig.
I)  in Fig.
in Fig.
II)  in Fig.
in Fig.
III) and  in Fig.
in Fig.
IV) then which curves are correct
I) 
II) 
III) 
IV) 
chemistry-General
chemistry-
Distribution of molecules with velocity is represented by the curve

Velocity corresponding to point  is
is
Distribution of molecules with velocity is represented by the curve

Velocity corresponding to point  is
is
chemistry-General
physics-
Fig., here shows P and Q as two equally intense coherent sources emitting radiations of wavelength 20m The separation PQ is 5m, and phase of P is ahead of the phase of Q by 90o A, B and C are three distant points of observation equidistant from the mid-point of PQ The intensity of radiations of A, B, C will bear the ratio

Fig., here shows P and Q as two equally intense coherent sources emitting radiations of wavelength 20m The separation PQ is 5m, and phase of P is ahead of the phase of Q by 90o A, B and C are three distant points of observation equidistant from the mid-point of PQ The intensity of radiations of A, B, C will bear the ratio

physics-General
physics-
Two identical narrow slits  are illuminated by light of wavelength
are illuminated by light of wavelength  from a point source P If, as shown in the diagram above the light is then allowed to fall on a screen, and if n is a positive integer, the condition for destructive interference at Q is that
from a point source P If, as shown in the diagram above the light is then allowed to fall on a screen, and if n is a positive integer, the condition for destructive interference at Q is that

Two identical narrow slits  are illuminated by light of wavelength
are illuminated by light of wavelength  from a point source P If, as shown in the diagram above the light is then allowed to fall on a screen, and if n is a positive integer, the condition for destructive interference at Q is that
from a point source P If, as shown in the diagram above the light is then allowed to fall on a screen, and if n is a positive integer, the condition for destructive interference at Q is that

physics-General
physics-
A ray of light of intensity I is incident on a parallel glass slab at a point A as shown It undergoes partial reflection and refraction At each reflection 25% of incident energy is reflected The rays AB and A’ B’ undergo interference The ratio 

A ray of light of intensity I is incident on a parallel glass slab at a point A as shown It undergoes partial reflection and refraction At each reflection 25% of incident energy is reflected The rays AB and A’ B’ undergo interference The ratio 

physics-General



