Maths-
General
Easy
Question
The number of ways to fill each of the four cells of the table with a distinct natural number such that the sum of the numbers is 10 and the sums of the numbers placed diagonally are equal, is-

- 8
- 4!
- 24
- None of these

The number of ways to fill each of the four cells of the table with a distinct natural number = Total ways of filling (x, b)  total ways of of filling (y, a)
total ways of of filling (y, a)

The correct answer is: 8
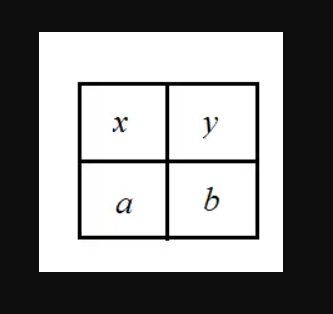
According to question x + b = a + y and x+ y+ a+ b=10
So x + b= 5 and a + y= 5
Given: all number should be different and two pairs whose sum is 5.
We have 2 pair of natural number that is (1,4),(2,3) whose sum is 5
We can choose 1 pair among 2 of them for (x, b) by 2! ways and they can rearranged in 2! ways.
So total ways for (x, b) will be 2!×2!
Remaining one pair will be for (a, y) and they can be arranged by 2! ways.
So total ways for (y, a) will be 2!.
Combining both situation together we get 2! × 2! × 2!= 8
Related Questions to study
physics-
ideal gas at 27°C is Compressed adiabatically, to  of its original Volume. If
of its original Volume. If  , then the rise in temperature is
, then the rise in temperature is
ideal gas at 27°C is Compressed adiabatically, to  of its original Volume. If
of its original Volume. If  , then the rise in temperature is
, then the rise in temperature is
physics-General
physics-
An ideal gas is taken through the cycle A B
B C
C A as shown in the figure, If the net heat supplied to the gas in the cycle is
A as shown in the figure, If the net heat supplied to the gas in the cycle is  , the work done by the gas in the process C
, the work done by the gas in the process C A is
A is

An ideal gas is taken through the cycle A B
B C
C A as shown in the figure, If the net heat supplied to the gas in the cycle is
A as shown in the figure, If the net heat supplied to the gas in the cycle is  , the work done by the gas in the process C
, the work done by the gas in the process C A is
A is

physics-General
physics-
Acyclic Process ABCD is Shown in the P V diagam. which of the following curves represent the same Represent the same Process?
V diagam. which of the following curves represent the same Represent the same Process?

Acyclic Process ABCD is Shown in the P V diagam. which of the following curves represent the same Represent the same Process?
V diagam. which of the following curves represent the same Represent the same Process?

physics-General
physics-
An ideal gas is taken through cyclic process as shown in the figure. The net work done by the gas is

An ideal gas is taken through cyclic process as shown in the figure. The net work done by the gas is

physics-General
physics-
Figure shows four P V diagrams which of these curves represent. isothermal and adiabatic processes?
V diagrams which of these curves represent. isothermal and adiabatic processes?

Figure shows four P V diagrams which of these curves represent. isothermal and adiabatic processes?
V diagrams which of these curves represent. isothermal and adiabatic processes?

physics-General
physics-
A thermodynamic system goes from States
(i)  to 2P,V
to 2P,V
(ii)  to P,2 V. Then what is work done in the two Cases.
to P,2 V. Then what is work done in the two Cases.
A thermodynamic system goes from States
(i)  to 2P,V
to 2P,V
(ii)  to P,2 V. Then what is work done in the two Cases.
to P,2 V. Then what is work done in the two Cases.
physics-General
physics-
When a System is taken from State I to State falling the path if, it is found that Q=70 cal and w =30 cal, along the path ibf Q=52c al. W atoug the path if is

When a System is taken from State I to State falling the path if, it is found that Q=70 cal and w =30 cal, along the path ibf Q=52c al. W atoug the path if is

physics-General
Maths-
If  and
and  , then
, then 
If  and
and  , then
, then 
Maths-General
Maths-
If 

 then
then 
If 

 then
then 
Maths-General
Maths-
If  , then
, then 
If  , then
, then 
Maths-General
physics-
Wafer of volume 2 filter in a container is heated with a coil of 1kw at 27°C. The lid of the containes is open and energy dissipates at the late of  . In how much time temperature will rise from to 27°C 77°C. Specific heat of wafers is
. In how much time temperature will rise from to 27°C 77°C. Specific heat of wafers is 
Wafer of volume 2 filter in a container is heated with a coil of 1kw at 27°C. The lid of the containes is open and energy dissipates at the late of  . In how much time temperature will rise from to 27°C 77°C. Specific heat of wafers is
. In how much time temperature will rise from to 27°C 77°C. Specific heat of wafers is 
physics-General
physics-
Starting with the same initial Conditions, an ideal gas expands from Volume  to
to  in three different ways. The Work done by the gas is
in three different ways. The Work done by the gas is  if the process is purely isothermal,
if the process is purely isothermal,  if purely isobasic and
if purely isobasic and  if purely adiabatic Then
if purely adiabatic Then

Starting with the same initial Conditions, an ideal gas expands from Volume  to
to  in three different ways. The Work done by the gas is
in three different ways. The Work done by the gas is  if the process is purely isothermal,
if the process is purely isothermal,  if purely isobasic and
if purely isobasic and  if purely adiabatic Then
if purely adiabatic Then

physics-General
physics-
A given mass of a gas expands from state A to B by three different paths 1,2 and 3 as shown in the figure. If  and
and  respectively be the work done by the gas along the three paths, then
respectively be the work done by the gas along the three paths, then

A given mass of a gas expands from state A to B by three different paths 1,2 and 3 as shown in the figure. If  and
and  respectively be the work done by the gas along the three paths, then
respectively be the work done by the gas along the three paths, then

physics-General
chemistry-
The enthalpy change at 298K in successive breaking of O–H bonds of water are H2O→H(g)+OH(g);ΔH=498KJmol–1 OH(g)→H(g)+O(g);ΔH=428KJmol–1 the bond enthalpy of O–H bond is-
The enthalpy change at 298K in successive breaking of O–H bonds of water are H2O→H(g)+OH(g);ΔH=498KJmol–1 OH(g)→H(g)+O(g);ΔH=428KJmol–1 the bond enthalpy of O–H bond is-
chemistry-General
chemistry-
The enthalpy change for the reaction H2(g)+C2H4(g)→C2H6(g)is........The bond energies are,H–H=103,C–H=99,C–C=80&C=C=145Kcalmol–1
The enthalpy change for the reaction H2(g)+C2H4(g)→C2H6(g)is........The bond energies are,H–H=103,C–H=99,C–C=80&C=C=145Kcalmol–1
chemistry-General



