Maths-
General
Easy
Question
The rank of  is
is
- 4
- 3
- 2
- 1
The correct answer is: 3
Related Questions to study
Maths-
The system of equations  has no solution, if
has no solution, if  is
is
The system of equations  has no solution, if
has no solution, if  is
is
Maths-General
chemistry-
The structure of the major product formed in the following reaction is

The structure of the major product formed in the following reaction is

chemistry-General
chemistry-
Identify the compound Y in the following reaction

Identify the compound Y in the following reaction

chemistry-General
chemistry-
Which of the following is halogen exchange reaction?
Which of the following is halogen exchange reaction?
chemistry-General
chemistry-
(CH3)2CHCl + NaI →(CH3)2CHI + NaCl The above reaction is known as
(CH3)2CHCl + NaI →(CH3)2CHI + NaCl The above reaction is known as
chemistry-General
chemistry-
Which of the following pairs are correctly matched?

Which of the following pairs are correctly matched?

chemistry-General
chemistry-
Which one is a nucleophilic substitution reaction among the following?
Which one is a nucleophilic substitution reaction among the following?
chemistry-General
chemistry-
The major product in the reaction is

The major product in the reaction is

chemistry-General
chemistry-
In the following reaction : 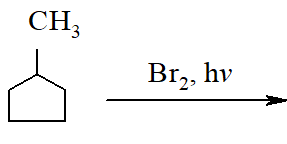 The major product obtained is
The major product obtained is
In the following reaction : 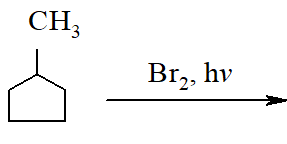 The major product obtained is
The major product obtained is
chemistry-General
chemistry-
The reagents for the following conversions is / are

The reagents for the following conversions is / are

chemistry-General
chemistry-
In the reaction R – OH + HX → R – X + H2O, The reactivity of different alcohols is
In the reaction R – OH + HX → R – X + H2O, The reactivity of different alcohols is
chemistry-General
Maths-
If  is a square matrix of order 3 then
is a square matrix of order 3 then  Adj
Adj 
If  is a square matrix of order 3 then
is a square matrix of order 3 then  Adj
Adj 
Maths-General
chemistry-
Which of the following compounds will undergo racemization when solution of KOH hydrolyses?
i) 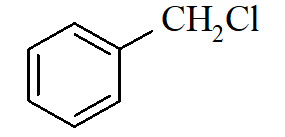
ii) CH3CH2CH2Cl
iii) 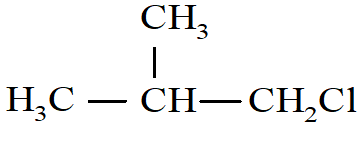
iv) 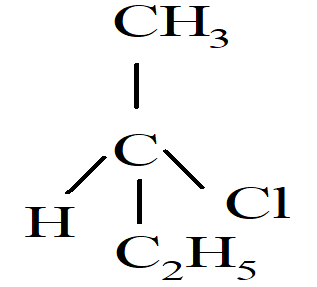 </span
</span
Which of the following compounds will undergo racemization when solution of KOH hydrolyses?
i) 
ii) CH3CH2CH2Cl
iii) 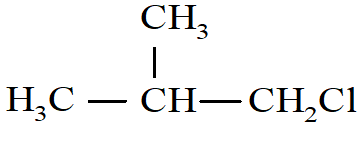
iv)  </span
</span
chemistry-General
physics-
An aero plane moving horizontally at a speed of  and at a height of
and at a height of  is to drop a bomb on a target. At what horizontal distance from the target should the bomb be released
is to drop a bomb on a target. At what horizontal distance from the target should the bomb be released
An aero plane moving horizontally at a speed of  and at a height of
and at a height of  is to drop a bomb on a target. At what horizontal distance from the target should the bomb be released
is to drop a bomb on a target. At what horizontal distance from the target should the bomb be released
physics-General
chemistry-
Which of the carbon atoms present in the molecule given below are asymmetric?

Which of the carbon atoms present in the molecule given below are asymmetric?

chemistry-General



