Physics-
General
Easy
Question
A photodetector is made from a semiconductor In  As with
As with  . What is the maximum wavelength, which it can detect
. What is the maximum wavelength, which it can detect
- 1000 nm
- 1703 nm
- 500 nm
- 173 nm
The correct answer is: 1703 nm
Related Questions to study
chemistry-
For Li+2,r2:r5 will be-
For Li+2,r2:r5 will be-
chemistry-General
chemistry-
When the atoms of gold sheet are bombarded with a be am of α-particles, only a few α- particles get deflected where as most of them go straight un deflected. This is because–
When the atoms of gold sheet are bombarded with a be am of α-particles, only a few α- particles get deflected where as most of them go straight un deflected. This is because–
chemistry-General
chemistry-
Mass of neutron is........times the mass of electron -
Mass of neutron is........times the mass of electron -
chemistry-General
chemistry-
Which has highest e/m ratio–
Which has highest e/m ratio–
chemistry-General
chemistry-
At room temperature, sodium crystal lises in a body centred cubic cell with a=4.24Å. The the oretical density of sodium is–(Atomic mass of sodium=23.0gmol–1)
At room temperature, sodium crystal lises in a body centred cubic cell with a=4.24Å. The the oretical density of sodium is–(Atomic mass of sodium=23.0gmol–1)
chemistry-General
General
________ is a sudden shaking of the Earth’s crust.
________ is a sudden shaking of the Earth’s crust.
GeneralGeneral
chemistry-
For the structure given below the site marked as S is a :

For the structure given below the site marked as S is a :

chemistry-General
chemistry-
A compound alloy of gold and copper crystallizes in a cube lattice in which the gold atoms occupy the corners of a cube and the copper atoms occupy the centres of each of the cube faces. The formula of this compound is-
A compound alloy of gold and copper crystallizes in a cube lattice in which the gold atoms occupy the corners of a cube and the copper atoms occupy the centres of each of the cube faces. The formula of this compound is-
chemistry-General
chemistry-
What is the simplest formula of a solid whose cubic unit cell has the atom A ateach corner, the atom Bat each face centre and a Catom at the body centre-
What is the simplest formula of a solid whose cubic unit cell has the atom A ateach corner, the atom Bat each face centre and a Catom at the body centre-
chemistry-General
physics-
An equiconvex lens is cut into two halves along (i) XOX¢ and (ii) YOY¢ as shown in the figure. Let f, f ¢, f ¢¢ be the focal lengths of the complete lens, of each half in case (i), and of each half in case (ii), respectively
Choose the correct statement from the following

An equiconvex lens is cut into two halves along (i) XOX¢ and (ii) YOY¢ as shown in the figure. Let f, f ¢, f ¢¢ be the focal lengths of the complete lens, of each half in case (i), and of each half in case (ii), respectively
Choose the correct statement from the following

physics-General
physics-
A point object O is placed in front of a glass rod having spherical end of radius of curvature 30 cm. The image would be formed at

A point object O is placed in front of a glass rod having spherical end of radius of curvature 30 cm. The image would be formed at

physics-General
physics-
A convex lens is made up of three different materials as shown in the figure. For a point object placed on its axis, the number of images formed are
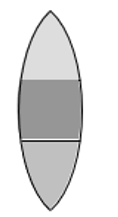
A convex lens is made up of three different materials as shown in the figure. For a point object placed on its axis, the number of images formed are

physics-General
chemistry-
In FCC crystal, which of the following shaded planes contains the following type of arrangement of atoms

In FCC crystal, which of the following shaded planes contains the following type of arrangement of atoms

chemistry-General
chemistry-
Assertion: The value of Vander Waal constant a is higher for NH3 than for N2.
Reason: Hydrogen bonding is present in ammonia.
Assertion: The value of Vander Waal constant a is higher for NH3 than for N2.
Reason: Hydrogen bonding is present in ammonia.
chemistry-General
chemistry-
If 10-4 dm-3 of water is introduced into a1.0 dm-3 flask at 300K,how many moles of water are in the vapour phase when equilibrium is established? (Given : Vapour pressure of H2O at300 is3170pa;R=8.314JK 1mol)
If 10-4 dm-3 of water is introduced into a1.0 dm-3 flask at 300K,how many moles of water are in the vapour phase when equilibrium is established? (Given : Vapour pressure of H2O at300 is3170pa;R=8.314JK 1mol)
chemistry-General



