Question
The first heart sound is
'Lubb' sound at the end of systole
'Dub' sound at the end of systole
'Lubb' sound at the beginning of systole
'Dub' sound at the beginning of systole
'Lubb' sound at the end of systole
'Dub' sound at the end of systole
'Lubb' sound at the beginning of systole
'Dub' sound at the beginning of systole
The correct answer is: 'Lubb' sound at the beginning of systole
Related Questions to study
 The compound (C) is:
The compound (C) is:
 The compound (C) is:
The compound (C) is:
The pressure-volume of varius thermodynamic processes is shown in graphs:

Work is the mole of transference of energy. It has been observed that reversible work done by the system is the maximum obtainable work

The works of isothermal and adiabatic processes are different from each other



If  and
and  are work done in isothermal, adiabatic, isobaric, and isochoric reversible processes, respectively then the correct sequence (for expansion) would be
are work done in isothermal, adiabatic, isobaric, and isochoric reversible processes, respectively then the correct sequence (for expansion) would be
The pressure-volume of varius thermodynamic processes is shown in graphs:

Work is the mole of transference of energy. It has been observed that reversible work done by the system is the maximum obtainable work

The works of isothermal and adiabatic processes are different from each other



If  and
and  are work done in isothermal, adiabatic, isobaric, and isochoric reversible processes, respectively then the correct sequence (for expansion) would be
are work done in isothermal, adiabatic, isobaric, and isochoric reversible processes, respectively then the correct sequence (for expansion) would be
The nerve like modified muscle in the right auricle is known as
The nerve like modified muscle in the right auricle is known as
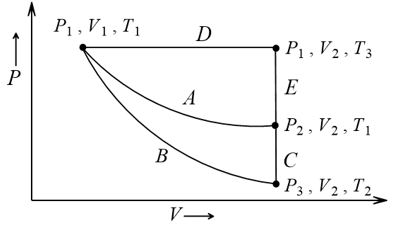
 Process
Process  represents
represents
 Process
Process  represents
represents
A: Excess amount of CO, in air is responsible for greenhouse effect.
R: CO2 is largely produced in respiratory functions.
A: Excess amount of CO, in air is responsible for greenhouse effect.
R: CO2 is largely produced in respiratory functions.
If a,b,c are in A.P the  are in
are in
If θ is one of the acute angles in a triangle, then the sine of theta is the ratio of the opposite side to the hypotenuse, the cosine is the ratio of the adjacent side to the hypotenuse, and the tangent is the ratio of the opposite side to the adjacent side.
If a,b,c are in A.P the  are in
are in
If θ is one of the acute angles in a triangle, then the sine of theta is the ratio of the opposite side to the hypotenuse, the cosine is the ratio of the adjacent side to the hypotenuse, and the tangent is the ratio of the opposite side to the adjacent side.
Systemic heart refers to
Systemic heart refers to
Which is a analgesic drug
Which is a analgesic drug
Penicillin was discovered by -
Penicillin was discovered by -
If  then
then
All three sides are equal. All three angles are congruent and are equal to 60 degrees. It is a regular polygon with three sides. The perpendicular drawn from vertex of the equilateral triangle to the opposite side bisects it into equal halves.
If  then
then
All three sides are equal. All three angles are congruent and are equal to 60 degrees. It is a regular polygon with three sides. The perpendicular drawn from vertex of the equilateral triangle to the opposite side bisects it into equal halves.
Two bodies of mass 1 kg and 3 kg have position vector  respectively the center of mass of this system has a position vector.
respectively the center of mass of this system has a position vector.
Two bodies of mass 1 kg and 3 kg have position vector  respectively the center of mass of this system has a position vector.
respectively the center of mass of this system has a position vector.
In 
In 
In HCl macule the separation between the nuclei of the two atoms is about The approximate location of the center of mass of the molecule is - -A,i with respect of Hydrogen atom ( mass of is 35.5 times of mass of Hydrogen)
In HCl macule the separation between the nuclei of the two atoms is about The approximate location of the center of mass of the molecule is - -A,i with respect of Hydrogen atom ( mass of is 35.5 times of mass of Hydrogen)