Chemistry-
General
Easy
Question
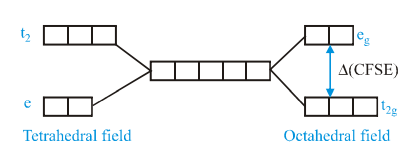
When degenerate d-orbitals of an iSolated atom/ion come under influence of magnetic field of ligands, the degeneracy is lost The two set  and
and  are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed
are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed  diagrammatically as:
diagrammatically as:
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for  is about 4/9 times to
is about 4/9 times to  tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colour Such transitions are not possible with d0 and d10 configuration.
tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colour Such transitions are not possible with d0 and d10 configuration.
For an octahedral complex, which of the following d-electron configuration will give maximum CFSE?
- High spin d6
- Low spin d4
- Low spin d5
- High spin d7
The correct answer is: Low spin d5
Related Questions to study
chemistry-
When degenerate d-orbitals of an iSolated atom/ion come under influence of magnetic field of ligands, the degeneracy is lost The two set  and
and  are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as:
are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as: 
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for  is about 4/9 times to
is about 4/9 times to  tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colour Such transitions are not possible with d0 and d10 configuration.
tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colour Such transitions are not possible with d0 and d10 configuration.
The d-orbitals, which are stabilised in an octahedral magnetic field, are –
When degenerate d-orbitals of an iSolated atom/ion come under influence of magnetic field of ligands, the degeneracy is lost The two set  and
and  are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as:
are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as: 
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for  is about 4/9 times to
is about 4/9 times to  tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colour Such transitions are not possible with d0 and d10 configuration.
tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colour Such transitions are not possible with d0 and d10 configuration.
The d-orbitals, which are stabilised in an octahedral magnetic field, are –
chemistry-General
chemistry-
hen degenerate d-orbitals of an iSolated atom/ion come under influence of magnetic field of ligands, the degeneracy is lost The two set  and
and  are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as:
are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as:
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for  is about 4/9 times to
is about 4/9 times to  tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colourSuch transitions are not possible with d0 and d10 configuration.
tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colourSuch transitions are not possible with d0 and d10 configuration.
The CFSE for  complex is 18000 cm–1The
complex is 18000 cm–1The  for
for  will be –
will be –
hen degenerate d-orbitals of an iSolated atom/ion come under influence of magnetic field of ligands, the degeneracy is lost The two set  and
and  are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as:
are either stabilized or destrabilized depending upon the nature of magnetic fieldIt can be expressed diagrammatically as:

Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for  is about 4/9 times to
is about 4/9 times to  tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colourSuch transitions are not possible with d0 and d10 configuration.
tetrahedral complexes, (CFSE for octahedral complex)This energy lies in visible region and i.e., why electronic transition are responsible for colourSuch transitions are not possible with d0 and d10 configuration.
The CFSE for  complex is 18000 cm–1The
complex is 18000 cm–1The  for
for  will be –
will be –
chemistry-General
chemistry-
Double salts are addition compounds which lose their identity in aqueous Solution whereas complexes which are also addition compounds do not lose their identity in aqueous Solution. The coordination compounds show isomerism and find applications in photography, qualitative analysis, metallurgy, water purification and in the treatment of various diseases.
Which of the following statements is incorrect ?
Double salts are addition compounds which lose their identity in aqueous Solution whereas complexes which are also addition compounds do not lose their identity in aqueous Solution. The coordination compounds show isomerism and find applications in photography, qualitative analysis, metallurgy, water purification and in the treatment of various diseases.
Which of the following statements is incorrect ?
chemistry-General
chemistry-
Statement-I : EAN of Fe in ferrocene is 36
Statement-II : 6 e – are co-ordinated by each cyclo pentadien ring with central metal ion.
e – are co-ordinated by each cyclo pentadien ring with central metal ion.
Statement-I : EAN of Fe in ferrocene is 36
Statement-II : 6 e – are co-ordinated by each cyclo pentadien ring with central metal ion.
e – are co-ordinated by each cyclo pentadien ring with central metal ion.
chemistry-General
chemistry-
Which of the following ions are optically active?
I) 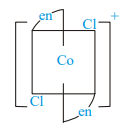
II) 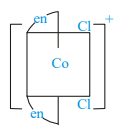
III) 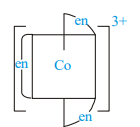
IV) 
Which of the following ions are optically active?
I) 
II) 
III) 
IV) 
chemistry-General
chemistry-
Of the following configurations, the optical isomers are :
I) 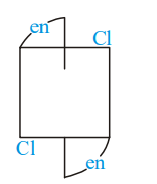
II) 
III) 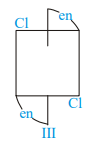
IV) 
Of the following configurations, the optical isomers are :
I) 
II) 
III) 
IV) 
chemistry-General
chemistry-
The complexes given below show : 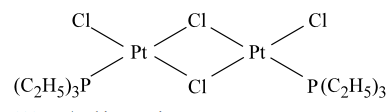 and
and 
The complexes given below show :  and
and 
chemistry-General
maths-
Suppose that the number of telephone calls coming into a telephone exchange between 10 A.M. and 11A.M., say, X1 is a random variable with poison distribution with parameter 2. Similarly the number of calls arriving between 11 A.M. and 12 noon, say X2 also follows a poison distribution with parameter 6. If X1 and X2 are independent, the probability that more than 5 calls come in between 10 A.M. and 12 noon is
Suppose that the number of telephone calls coming into a telephone exchange between 10 A.M. and 11A.M., say, X1 is a random variable with poison distribution with parameter 2. Similarly the number of calls arriving between 11 A.M. and 12 noon, say X2 also follows a poison distribution with parameter 6. If X1 and X2 are independent, the probability that more than 5 calls come in between 10 A.M. and 12 noon is
maths-General
chemistry-
The van der Waals constant for gases and
and  are as follows:
are as follows:

Which gas has the highest critical temperature?
The van der Waals constant for gases and
and  are as follows:
are as follows:

Which gas has the highest critical temperature?
chemistry-General
chemistry-
The distribution of the molecular velocities of gas molecules at any temperature  is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)
is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)

Where
 is molecular velocity
is molecular velocity
 is number of molecules having velocity
is number of molecules having velocity 
Let us define  , which is equal to the number of molecules between the velocity range
, which is equal to the number of molecules between the velocity range  and
and  , given by
, given by

Where
 is total number of molecules
is total number of molecules
 and
and 
 is universal gas constant
is universal gas constant
 is temperature of the gas
is temperature of the gas
 is molecular weight of the gas
is molecular weight of the gas
SI units of  are
are
The distribution of the molecular velocities of gas molecules at any temperature  is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)
is shown below. (The plot below is known as Maxwell’s distribution of molecular speeds.)

Where
 is molecular velocity
is molecular velocity
 is number of molecules having velocity
is number of molecules having velocity 
Let us define  , which is equal to the number of molecules between the velocity range
, which is equal to the number of molecules between the velocity range  and
and  , given by
, given by

Where
 is total number of molecules
is total number of molecules
 and
and 
 is universal gas constant
is universal gas constant
 is temperature of the gas
is temperature of the gas
 is molecular weight of the gas
is molecular weight of the gas
SI units of  are
are
chemistry-General
chemistry-
The figure given below shows three glass chambers that are connected by valves of negligible volume. At the outset of an experiment, the valves are closed and the chambers contain the gases as detailed in the diagram. All the chambers are at the temperature of 300 K and external pressure of 1.0 atm
 atm
atm

What will be the work done by  gas when valve 2 is opened and value 1 remains closed?
gas when valve 2 is opened and value 1 remains closed?
The figure given below shows three glass chambers that are connected by valves of negligible volume. At the outset of an experiment, the valves are closed and the chambers contain the gases as detailed in the diagram. All the chambers are at the temperature of 300 K and external pressure of 1.0 atm
 atm
atm

What will be the work done by  gas when valve 2 is opened and value 1 remains closed?
gas when valve 2 is opened and value 1 remains closed?
chemistry-General
chemistry-
In simple cubic lattice, the spheres are packed in the form of a square array by laying down a base of spheres and then piling upon the base other layers in such a way that each sphere is immediately above the other sphere. In this structure, each sphere is in contact with six nearest neighbours. The percentage of occupied volume in this structure can be calculated as follows
The edge length ‘ ’ of the cube will be twice the radius of the sphere,
’ of the cube will be twice the radius of the sphere,  . Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is
. Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is

 or
or 
The fraction of the total volume occupied by the sphere is

In a simple cubic cell, an atom at the corner contributes to the unit cell
In simple cubic lattice, the spheres are packed in the form of a square array by laying down a base of spheres and then piling upon the base other layers in such a way that each sphere is immediately above the other sphere. In this structure, each sphere is in contact with six nearest neighbours. The percentage of occupied volume in this structure can be calculated as follows
The edge length ‘ ’ of the cube will be twice the radius of the sphere,
’ of the cube will be twice the radius of the sphere,  . Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is
. Since, in the primitive cubic lattice, there is only one sphere present in the unit lattice, the volume occupied by the sphere is

 or
or 
The fraction of the total volume occupied by the sphere is

In a simple cubic cell, an atom at the corner contributes to the unit cell
chemistry-General
chemistry-
Sketch shows the plot of Zvsp for a hypothetical gas for one mole at
three distinct temperature


Boyle’s temperature is the temperature at which a gas shows ideal behaviour over a pressure range in the low pressure region. Boyle’s temperature  . If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as
. If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as  and the temperature above critical temperature curve is more like
and the temperature above critical temperature curve is more like at
at 
For 500 K plot value of  changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of
changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of  will be
will be
Sketch shows the plot of Zvsp for a hypothetical gas for one mole at
three distinct temperature


Boyle’s temperature is the temperature at which a gas shows ideal behaviour over a pressure range in the low pressure region. Boyle’s temperature  . If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as
. If a plot is obtained at temperature below Boyle’s temperature then the curve will show negative deviation in low pressure region and positive deviation in the high pressure region. Near critical temperature, the curve is more likely as  and the temperature above critical temperature curve is more like
and the temperature above critical temperature curve is more like at
at 
For 500 K plot value of  changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of
changes from 2 to 2.2 if pressure is varied from 1000 atm to 1200 atm (high pressure) then the value of  will be
will be
chemistry-General
physics-
In double slit experiment, the distance between two slits is 0.6 mm and these are illuminated with light of wavelength 4800 A0 The angular width of dark fringe on the screen at a distance 120 cm from slits will be
In double slit experiment, the distance between two slits is 0.6 mm and these are illuminated with light of wavelength 4800 A0 The angular width of dark fringe on the screen at a distance 120 cm from slits will be
physics-General
chemistry-
I, II, and III are three isotherms, respectively, at  and
and  . Temperature will be in order
. Temperature will be in order

I, II, and III are three isotherms, respectively, at  and
and  . Temperature will be in order
. Temperature will be in order

chemistry-General



