Maths-
General
Easy
Question
When a die is rolled twice, if the event of getting an even number is denoted by a success and the number of successes as a random variable, then distribution and mean of the variate are
The correct answer is: 
Related Questions to study
chemistry-
Enthalpy of CH4+  O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
Enthalpy of CH4+  O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
O2→CH3OH is negative. If enthalpy of combustion of CH4 and CH3OH are x and y respectively. Then which relation is correct-
chemistry-General
chemistry-
Change in enthalpy for reaction 2H2O2(l) → 2H2O(l)+O2(g) If heat of formation of H2O2 (l) and H2O (l)are– 188&–286KJ/mol respectively
Change in enthalpy for reaction 2H2O2(l) → 2H2O(l)+O2(g) If heat of formation of H2O2 (l) and H2O (l)are– 188&–286KJ/mol respectively
chemistry-General
chemistry-
For there action C2H5OH(l)+3O2(g)→2CO2(g)+3H2O(l) Which one istrue -
For there action C2H5OH(l)+3O2(g)→2CO2(g)+3H2O(l) Which one istrue -
chemistry-General
chemistry-
2Zn+O2 3/2 →2ZnODG°=–616J 2Zn+S2→2ZnSDG°=–293J S2+2O2 3/4 →2SO2DG°=–408J DG°forthefollowingreactionis-2ZnS+3O2 3/4 →2ZnO+2SO
2Zn+O2 3/2 →2ZnODG°=–616J 2Zn+S2→2ZnSDG°=–293J S2+2O2 3/4 →2SO2DG°=–408J DG°forthefollowingreactionis-2ZnS+3O2 3/4 →2ZnO+2SO
chemistry-General
Maths-
A random variable has the following distribution.
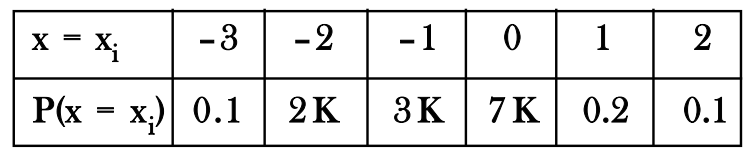
Then for the values, A = K, B = Mean, C = Variance, the ascending order is
A random variable has the following distribution.

Then for the values, A = K, B = Mean, C = Variance, the ascending order is
Maths-General
chemistry-
The heat of combustion of ethanolina bomb calorimeteris–670.48Kcalmol–1at25°C.Whatis DE at 25°C for the reaction?
The heat of combustion of ethanolina bomb calorimeteris–670.48Kcalmol–1at25°C.Whatis DE at 25°C for the reaction?
chemistry-General
Maths-
A random variable X has the following distribution
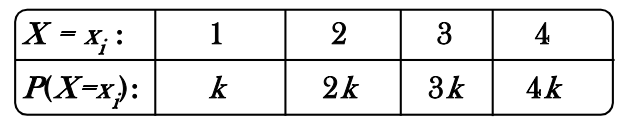
The value of  and P(X<3) are equal to
and P(X<3) are equal to
A random variable X has the following distribution

The value of  and P(X<3) are equal to
and P(X<3) are equal to
Maths-General
Maths-
If m and 2 s are the mean and variance of the random variable X, whose distribution is given by

If m and 2 s are the mean and variance of the random variable X, whose distribution is given by

Maths-General
Maths-
The distribution of a random variable X is given below
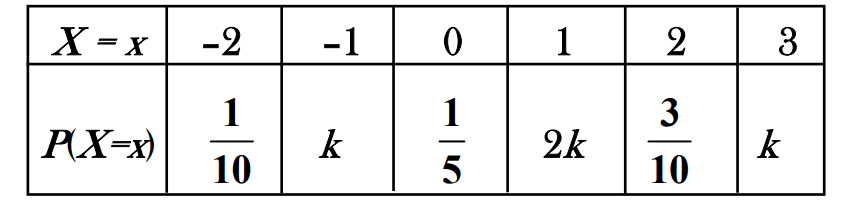
The value of k is
The distribution of a random variable X is given below

The value of k is
Maths-General
maths-
The number of different triangles formed by joining the points A, B, C, D, E, F and G as shown in the figure given below is

The number of different triangles formed by joining the points A, B, C, D, E, F and G as shown in the figure given below is

maths-General
maths-
The number of ways that the letters of the word ”PERSON” can be placed in the squares of the adjoining figure so that no row remains empty
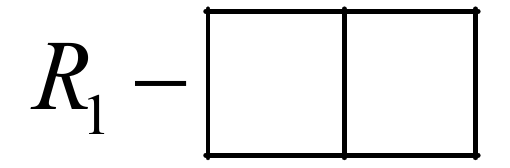
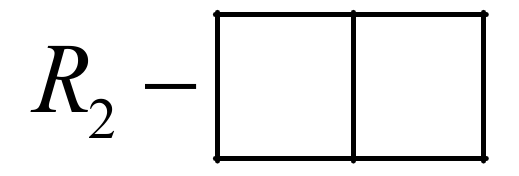

The number of ways that the letters of the word ”PERSON” can be placed in the squares of the adjoining figure so that no row remains empty



maths-General
maths-
The value of  is
is
The value of  is
is
maths-General
maths-
The determinant  is equal to zero if
is equal to zero if  are in
are in
The determinant  is equal to zero if
is equal to zero if  are in
are in
maths-General
Maths-
If  satisfy
satisfy  then
then 
If  satisfy
satisfy  then
then 
Maths-General
chemistry-
End product of the following sequence of reaction is :

End product of the following sequence of reaction is :

chemistry-General



