General
General
Easy
Question
"The sky appears blue. This is due to atmospheric refraction.”
Identify whether the above statement is true or false.
- True
- False

Blue is scattered more than other colors
The correct answer is 'False'.
Sky appears blue because of Scattering.Blue light is scattered in all directions by the tiny molecules of air in Earth's atmosphere. Blue is scattered more than other colors because it travels as shorter, smaller waves. This is why we see a blue sky most of the time.
So False
Related Questions to study
Physics-
A car is moving with a speed of 100 kmh-1 . if the mass of the car is 950 kg, then its kinetic energy is
A car is moving with a speed of 100 kmh-1 . if the mass of the car is 950 kg, then its kinetic energy is
Physics-General
Chemistry-
Acetic acid is a weak electrolyte because:
Acetic acid is a weak electrolyte because:
Chemistry-General
Physics-
A body is projected horizontally from the top of a high tower with a speed of 20 m/s. After 4 sec the displacement of the body is (take g=10m/s2)
A body is projected horizontally from the top of a high tower with a speed of 20 m/s. After 4 sec the displacement of the body is (take g=10m/s2)
Physics-General
Chemistry-
Consider the reaction: The rate equation for this reaction is :
The rate equation for this reaction is :  Which of these mechanisms is/are consistent with this rate equation ?
Which of these mechanisms is/are consistent with this rate equation ?
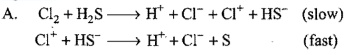

Consider the reaction: The rate equation for this reaction is :
The rate equation for this reaction is :  Which of these mechanisms is/are consistent with this rate equation ?
Which of these mechanisms is/are consistent with this rate equation ?
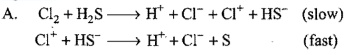

Chemistry-General
Physics-
Two particles move in a uniform gravitational field with an acceleration g. At the initial moment the particles were located at one point and moved with velocities V1 = 1 m/s and V2 = 4 m/s horizontally in opposite directions. The distance between the particles at the moment when their velocity vectors become mutually perpendicular is (g=10m/s2)
Two particles move in a uniform gravitational field with an acceleration g. At the initial moment the particles were located at one point and moved with velocities V1 = 1 m/s and V2 = 4 m/s horizontally in opposite directions. The distance between the particles at the moment when their velocity vectors become mutually perpendicular is (g=10m/s2)
Physics-General
Chemistry-
An endothermic reaction with high activation energy for the forward reaction is given by the diagram:
An endothermic reaction with high activation energy for the forward reaction is given by the diagram:
Chemistry-General
Chemistry-
Graph between concentration of the product' x' and time' t' for  is given ahead:
is given ahead:
 The graph between
The graph between  and time wil1 be of the type:
and time wil1 be of the type:
Graph between concentration of the product' x' and time' t' for  is given ahead:
is given ahead:
 The graph between
The graph between  and time wil1 be of the type:
and time wil1 be of the type:
Chemistry-General
Chemistry-
Following is the graph between  and time t for second order reaction.
and time t for second order reaction.  hence rate at the start of reaction will be:
hence rate at the start of reaction will be:

Following is the graph between  and time t for second order reaction.
and time t for second order reaction.  hence rate at the start of reaction will be:
hence rate at the start of reaction will be:

Chemistry-General
Chemistry-
Following is the graph between log tll2 and log a (a = initial concentration) for a given
 reaction at
reaction at  e. Hence, order is:
e. Hence, order is:
Following is the graph between log tll2 and log a (a = initial concentration) for a given
 reaction at
reaction at  e. Hence, order is:
e. Hence, order is:
Chemistry-General
Chemistry-
For the reaction  the probable mechanism is,
the probable mechanism is,
 The rate law will be:
The rate law will be:
For the reaction  the probable mechanism is,
the probable mechanism is,
 The rate law will be:
The rate law will be:
Chemistry-General
Chemistry-
A drop of solution (volume 0.05 mL) contains  mole of W. If the rate constant of disappearance of H+ is 107 mol
mole of W. If the rate constant of disappearance of H+ is 107 mol , how long would it take for W in the drop tell disappear?
, how long would it take for W in the drop tell disappear?
A drop of solution (volume 0.05 mL) contains  mole of W. If the rate constant of disappearance of H+ is 107 mol
mole of W. If the rate constant of disappearance of H+ is 107 mol , how long would it take for W in the drop tell disappear?
, how long would it take for W in the drop tell disappear?
Chemistry-General
Chemistry-
P4 + 5O2 ¾ → X  Y, Y is
Y, Y is
P4 + 5O2 ¾ → X  Y, Y is
Y, Y is
Chemistry-General
Chemistry-
Cu2+ + 2e– ¾® Cu; log[Cu2+] vs Ered graph is of the type shown in figure where OA = 0.34V, then electrode potential of the half cell of Cu/Cu2+ (0.1 M) will be

Cu2+ + 2e– ¾® Cu; log[Cu2+] vs Ered graph is of the type shown in figure where OA = 0.34V, then electrode potential of the half cell of Cu/Cu2+ (0.1 M) will be

Chemistry-General
Chemistry-
Which statement is correct for alkaline earth metals
Which statement is correct for alkaline earth metals
Chemistry-General
Chemistry-
The incorrect statement is
The incorrect statement is
Chemistry-General



